Moseley & Atomic Number
In
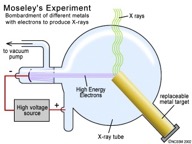
1913, Henry Mosley, the British chemist, found that by bombarding metals with
electrons, X-rays are emitted. It was
found that electrons were effecting the positive
charges in the nucleus and caused different frequencies of X-rays to be
emitted. Mosley was able to convert the
differences in the frequencies into whole numbers and called them the atomic
number. We now know that this number
represents the number of protons in the nucleus. This sequencing of atomic numbers allowed the
few errors in Mendeleev’s work to be fixed and supported his table as the most
accurate and useful chart; allowing it to be adopted as the standard periodic
table. Mendeleev’s model had trouble resolving
the three main errant sequences of Ar-K, Co-Ni & Te-I because they were arranged by atomic mass.
Though
Mendeleev had cobalt and nickel in the right order, further experimentation
showed that their atomic masses were incorrectly calculated during the 1800s
and they had to be switched. When argon
was discovered it appeared to fall between potassium
and calcium, though that would throw off the elements around calcium. Mosley’s work straightened out all of the problems.
The sequencing of the elements by increasing atomic number is known as
atomic law. With this new method of
testing, it was also easy to confirm Mendeleev’s hypotheses about missing
elements, validating his arrangement and making it the
standard for use in labs around the world.
Unfortunately, World War I broke out and Mosley’s promising career was
cut short when he died in the Battle of Gallipoli in 1915.

Next - Bohr/Nagaoka & the Solar System
Model