Rutherford/Marsden/Geiger and Empty Space
In the early twentieth century (published in 1911) a group of British scientists led by Ernest Rutherford were experimenting with radioactive chemicals and determined that there were three different types of radioactive particles. They found that the least energetic of the particles was an alpha (α, 42He) particle, the second most energetic, a beta (β, 0-1e) particle and the most energetic, a gamma (γ, 00γ) particle. All nuclear particles begin in the nucleus and burst forth, but it is possible to focus the particles into a beam. By focusing the beams through a magnetic field, Rutherford determined that the most massive particle, the alpha particle was attracted to the negatively charged plate and must therefore be positive, the beta particle was attracted to the positively charged plate and must be negative, and the gamma particles were unaffected by the magnetic field.
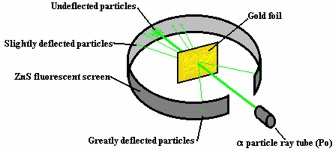
While working with alpha particles, Geiger and Marsden
discovered a truly remarkable fact-atoms are mostly empty space. The team was firing the alpha particles, from
a polonium source, into sheets of gold foil.
The gold foil was surrounded by a zinc sulfide (ZnS) screen that would
fluoresce when it was struck by the positively charged alpha particles. They hypothesized that, if the Thompson model
was correct, that the massive, energetic alpha particles would rip through the
thin pieces of gold with only minor deflection.
Early results showed that Thompson was correct, however, after further
testing it was found that, despite most of the particles passing through with
minor deflection as expected, several fluorescing spots appeared at sharp
angles and still others were reflected back toward the source.
The
only plausible explanation for these results would be that the atom must have a
compact, positively charged center and be composed primarily of empty space
where the electron can move around. The
positive center was given the title nucleus.
In 1917, Rutherford identified the positive particle as the proton. The results of the experiment were so
shocking that Rutherford stated that it was much like firing a 15” shell from a
Howitzer at a sheet of paper and having it bounce off. Rutherford’s research began the evolution of
the nuclear atom and was the earliest movement toward the abstract Quantum
Mechanical model that we use today.



Ernest Rutherford, Ernest Marsden and Hans Geiger
Rutherford’s Gold Foil Experiment shocked the world by
showing that solid was an illusion and matter was mostly empty space.

When Rutherford’s team of researchers came across the
fact that atoms were mostly empty space, they met with a lot of resistance from
the public. The idea that the stuff that
makes up “solids” was not how we thought it to be was very unsettling since we
could not see through things or pass our hands through them. The new concept of the atom can be
illustrated by the bail of fencing above.
If enough layers are stacked together, the bail becomes stronger and it
is more difficult to see through the large holes.
Next - Moseley & Atomic Numbers